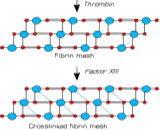
Factor XIII is a transglutaminase that circulates in the plasma as a heterotetramer of two catalytic A subunits and two carrier B subunits. When thrombin has converted fibrinogen to fibrin, the latter forms a proteinaceous network in which every E-unit is crosslinked to only one D-unit. Factor XIII is activated by thrombin into factor XIIIa; its activation into Factor XIIIa requires calcium as a cofactor. A cleavage by thrombin between residue Arg37 and Gly38 on the N-terminus of the A subunit, leads to the release of the activation peptide (MW 4000 da). In the presence of calcium the carrier subunits dissociate from the catalytic subunits, leading to a 3D change in conformation of factor XIII and hence the exposure of catalytic cysteine residue. Upon activation by thrombin, factor XIIIa acts on fibrin to form γ-glutamyl-Є-lysyl amide cross links between fibrin molecules to form an insoluble clot.